Strontium nitrate formula, also known as Strontium dinitrate formula or Nitrate de strontium formula is discussed in this article. It is an inorganic compound which is made of two elements viz nitrogen and strontium. The molecular or chemical formula of Strontium nitrate is Sr(NO3)2.
Strontium dinitrate is a crystalline solid white in colour. It is a noncombustible compound but enhances the burning of combustible compounds. It is widely used in pyrotechnics, to make chemicals, and in medicine.
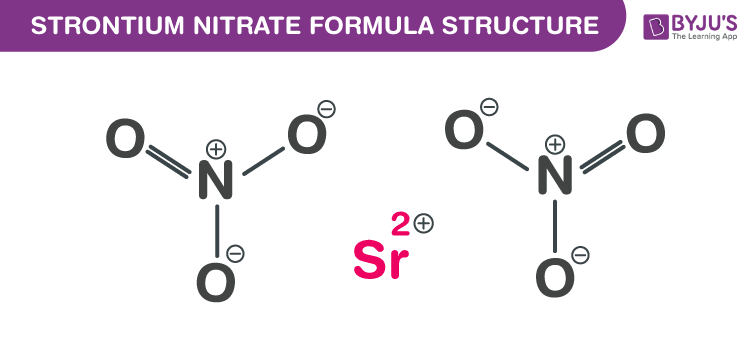
Strontium nitrate Formula Structure
Properties Of Strontium nitrate Formula
| Chemical formula | Sr(NO3)2 |
| Molecular weight | 211.630 g/mol (anhydrous)
283.69 g/mol (tetrahydrate) |
| Density | 2.986 g/cm3 (anhydrous)
2.20 g/cm3 (tetrahydrate) |
| Boiling point | 645 °C (anhydrous) |
| Melting point | 570 °C (anhydrous)
100 °C, decomposes (tetrahydrate) |
Nitrate de strontium can explode if excess quantities are involved in the fire. Also, if it is exposed to heat for a long duration then it can explode. When burnt it produces toxic oxides of nitrogen.
To learn more about Strontium nitrate formula from the expert faculties at BYJU’S, register now! Also, you can download notes on Nitrate de strontium for free.
Comments